- Lewis Dot Structure For Ionic Compounds Calculator
- Lewis Dot Structure Simulator
- Resonance Structure Calculator Chemistry
- Lewis Dot Structure Calculator
- Lewis Dot Structure Calculator
- Lewis Electron Dot Structure Calculator. Lewis structure calculator ot 680 can obd2 obd ii code:: 痞客邦 dot ionic bonds worksheet double printable worksheets and activities for teachers questions solved: select the atoms drawn with valid struct chegg com.
- A step-by-step explanation of how to draw the OBr2 Lewis Dot Structure. For the OBr2 structure use the periodic table to find the total number of valence ele.
A planar structure will be formed. 2) Si has 4 electrons its valence shell & Cl need only 1 electron to complete its octet, therefore 4 Cl will make 4 bonds with Si.A tetrahedral shape will be formed. 3) Be has 2 electrons in its valence shell & Fe has 2 electrons in its valence shell, so they make a planar structure.

Lewis Structures are important to learn because they help us predict:
- the shape of a molecule.
- how the molecule might react with other molecules.
- the physical properties of the molecule (like boiling point, surface tension, etc.).
That helps us understand and predict interactions with things like medicine and our body, materials used to make buildings and airplanes, and all sorts of other substances. Lewis structures don't tell us everything, but along with molecule geometry and polarity they are hugely informative.
| Search 100+ Lewis Structures on our site. (Opens new window.) |

Lewis Dot Structure For Ionic Compounds Calculator
Click the Chemical Formula to see the Lewis Structure Tropico 4 for mac torrent. Upon choosing a random island, a player may customize the size of the island, vegetation, mineral deposits, and elevation, as well as other game parameters. There are various expansion packs which give access to more islands.After choosing the island, the player may choose a premade avatar, some based on various Latin American and Caribbean leaders (such as Fidel Castro, Augusto Pinochet, 'Papa Doc' Duvalier), or they can create their own avatar. For custom avatars, players may choose: gender, costumes, skin tone, hat, hairstyle, accessories, mustache, beard, earrings, traits, quality, and rise to power.
| Steps for Writing Lewis Structures
Advanced Steps |
Examples: ClF3, SF4,XeH4
Examples: SO42-, N2O, XeO3
Notable Exceptions to the Octet Rule
- H only needs 2 valence electrons.
- Be and B don't need 8 valence electrons.
- S and P sometimes have more than 8 val. Electrons.
- Elements in Period Three, Four, etc (on the periodic table) can hold more than 8 valence electrons.
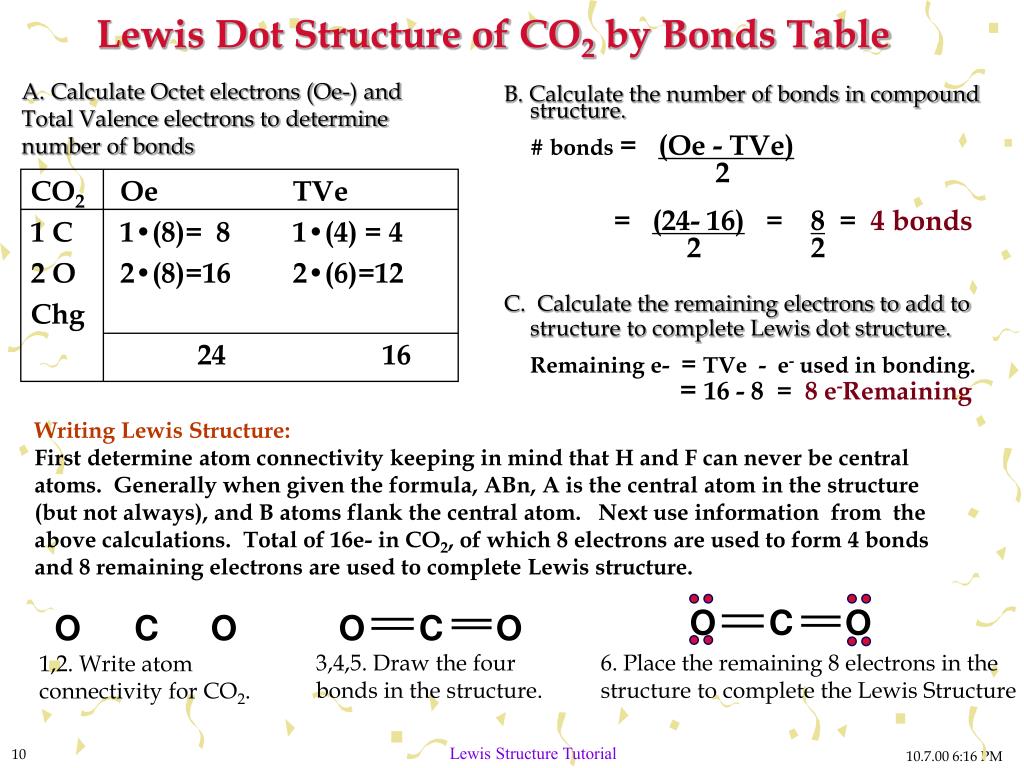
Commonly Tested Lewis Structures
Lewis Dot Structure Simulator
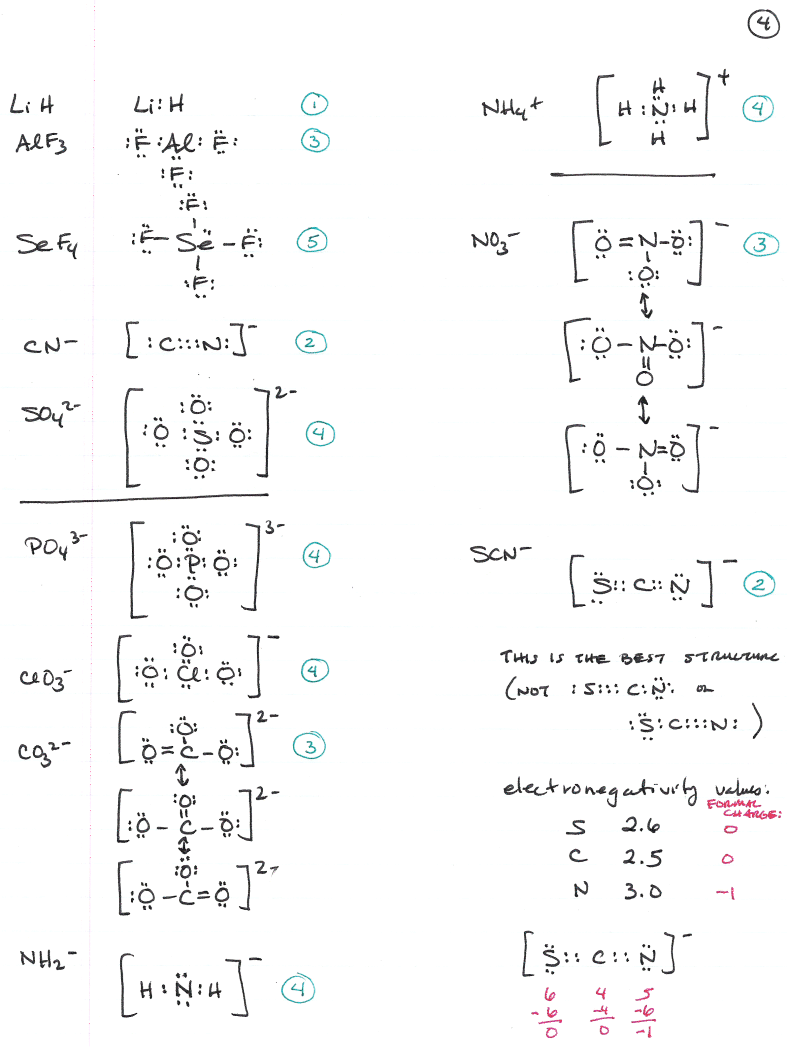
Lewis Structures are important to learn because they help us predict:
- the shape of a molecule.
- how the molecule might react with other molecules.
- the physical properties of the molecule (like boiling point, surface tension, etc.).
That helps us understand and predict interactions with things like medicine and our body, materials used to make buildings and airplanes, and all sorts of other substances. Lewis structures don't tell us everything, but along with molecule geometry and polarity they are hugely informative.
| Search 100+ Lewis Structures on our site. (Opens new window.) |
Lewis Dot Structure For Ionic Compounds Calculator
Click the Chemical Formula to see the Lewis Structure Tropico 4 for mac torrent. Upon choosing a random island, a player may customize the size of the island, vegetation, mineral deposits, and elevation, as well as other game parameters. There are various expansion packs which give access to more islands.After choosing the island, the player may choose a premade avatar, some based on various Latin American and Caribbean leaders (such as Fidel Castro, Augusto Pinochet, 'Papa Doc' Duvalier), or they can create their own avatar. For custom avatars, players may choose: gender, costumes, skin tone, hat, hairstyle, accessories, mustache, beard, earrings, traits, quality, and rise to power.
| Steps for Writing Lewis Structures
Advanced Steps Notable Exceptions to the Octet Rule
|
Commonly Tested Lewis Structures
Lewis Dot Structure Simulator
- Read my article in Science Education based on my dissertation.
We draw Lewis Structures to predict:
-the shape of a molecule.
-the reactivity of a molecule and how it might interact with other molecules.
-the physical properties of a molecule such as boiling point, surface tension, etc.
Framingham risk score canada. Video: Drawing the Lewis Structure for ICl4-
For the ICl4- Lewis structure the total number of valence electrons (found on the periodic table) for the ICl4- molecule. Once we know how many valence electrons there are in ICl4- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
In the Lewis structure of ICl4- there are total of 36 valence electrons.
Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons.
Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
It is helpful if you:
Resonance Structure Calculator Chemistry
- Try to draw the ICl4- Lewis structure before watching the video.
- Watch the video and see if you missed any steps or information.
- Try structures similar to ICl4- for more practice.
List of Lewis Structures
Lewis Dot Structure Calculator
| Acetone | BF3 | BH4- | BrF5 | BrO3- | C2H2 | C2H4 | ClO- |
| CH4 | ClO2- | ClO2 | ClO4- | CO | CS2 | H2O | H3O+ |
| HCl | HNO3 | I3- | ICl4- | IF5 | N2 | N3- | NH2OH |
| NH3 | NO2- | NO2 | NO3- | O2 | OF2 | PCl5 | PH3 |
| PO33- | PO43- | SCl2 | SF4 | SF6 | SO3 | SO42- | XeF2 |
| XeF4 |
